Overview of the research fields involved
This research seeks to advance the field of Photonics of thin films of novel polymers. The field of photonics is relatively new, developing primarily over the past 10 years as a novel alternative to electronics, in response to fundamental limitations encountered with the materials and design of electrical devices. The key to photonics is using light signals (photons) to transmit, store and process digital information, as opposed to electrical signals (electrons). One of the key advantages to optical systems is a much higher volume and efficiency of information that can be transmitted, since multiple light signals can co-exist in a material without interfering, and there is a wide range of 'coding variables' for photons (such as wavelength and polarization) necessarily unavailable to electrons. In addition to greater bandwidth in transmitted information, this subtle but key difference between photons and electrons also results in a much wider range of circuit architectures available for photonic processing devices, resulting in a vast performance potential for next-generation computers.
This innovation in information processing is also one of the materials used, as optimal platforms for photonics are usually not the traditional metals and silicon-based inorganics, but rather more easily-processed materials such as organic glasses and polymers. One of the most challenging aspects of information processing devices based on inorganic semiconductor electronic materials, is the extreme sensitivity of the chips to impurities such as dust and moisture. Indeed, much of the multi-billion dollar cost of recent chip fabrication facilities (such as that required for the Intel Pentium 4 processors, for example), is devoted to ensuring excruciatingly high standards of cleanliness during the tedious multi-step lithography. Alternative materials that are free from such requirements are hence very attractive.
One such paradigm that has received much recent attention is to mimic natural mechanisms of assembly of thin films and small-scale structure and function. These materials are generally referred to as 'self-assembly', meaning that an array of constituent components are spontaneously formed into the desired structures under proper conditions. One of the key advantages to this approach is that self-assembled systems then arrive at a thermodynamic minimum, and are hence very stable to de-construction or re-arrangement over time. Also due to this reason, the films are of significantly better coverage (fewer defects) than other non-equilibrium methods of thin film fabrication such as vapour deposition or spin-coating. Such natural examples of self-assembly include the folding of large protein polymers to activate their desired function, or the pairing and replication of DNA. An application of this natural self-assembly approach to thin films is the layer-by-layer sequential adsorption of charged polymers in aqueous solution. This highly innovative technique, co-developed in 1994 by Decher and Rubner, uses polymers that are water-soluble and contain charged ionic + or - groups, to self-assemble multilayer devices in beakers on the lab bench. In the short time since this new technique was reported, many systems have been demonstrated which contain useful electronic functional groups, such as conductive and electroluminescent polymers. Moreover, because these films are assembled in a water-based environment (much unlike all other electronic materials) these devices are, for the first time, able to be bio-compatible, and hence in principle are able to communicate with biological systems. To date however, there has been no work done with either the optical properties of these self-assembled multilayers, or in how light can be used to change the surface or structural properties important to biological systems.
The approach to research taken by the Barrett Group is to apply, for the first time, the emerging field of photonics to the new technique of self-assembly of multi-layer thin films, built from dilute solution. This novel approach to photonic devices will concentrate on the communication between light signals, and the bulk and surface structure of the films. Specifically, this program of research is an investigation of the optical and surface properties of thin films of novel polymers. The optical and surface properties are interrelated in these materials, allowing studies both of how light can be used to gather information about surfaces and structures, and how light can be used to influence surface and structural properties. This will be accomplished with polymer materials which incorporate both light-absorbing photo-active groups (azobenzene chromophores), and water-soluble ionic groups (electrolytes).
These azo-polyelectrolytes are thus very interesting as they have the unique capability to be both addressed with lasers as electro-optic materials (useful for information storage, holography, and signal processing devices for example), yet can also be self-assembled into structures on the molecular lengthscale, using the + and - ionic groups as building blocks. Furthermore, the aqueous nature of the materials will be used to explore compatibility between biological systems and more traditional electronic and photonic devices. An eventual goal of this research is to demonstrate that these bio-compatible films can be interfaced with current technologies and be used as bio-active sensors, to permit information processing systems to communicate directly with natural systems.
Nature of research
Research in the Barrett Group is strongly suited to multi-disciplinary research, and hence provides an excellent opportunity to train researchers in a number of different fields. Researchers in this group will obtain a fundamental grounding in physical polymer chemistry from first principles, and gain valuable training in specific techniques such as film and device preparation, polymer synthesis and characterization, geometric optics, surface science, electronics, and computer programming.
The optical techniques employed are not routine in any sense, but rather use an individually designed and assembled setup for each experiment. In many cases the hardware will be constructed, and software will be written by each researcher according to needs as they evolve. This design-from-scratch approach using basic optical and surface science components provides the best possible advanced training in basic experimental design and testing, and the versatility of the equipment allows for ready exploration into new research directions discovered or envisaged by each researcher. Furthermore, the intellectual exercise of critically designing and conducting most aspects of one's experiments is invaluable as basic academic and scientific training.
Multi-disciplinary research, such as that conducted in the Barrett Laboratory, thus presents an invaluable opportunity for researchers from a variety of backgrounds to gain exposure to areas of experiment and theory that they would not otherwise encounter. Many of the most important recent breakthroughs in materials and polymer chemistry have been the result of exploring the interface between traditionally separate areas, and this research program is ideally suited to continuing such novel investigations. One of the strengths of the a Polymer Photonics Laboratory is that in addition to specific skills honed, researchers trained will emerge as generalists, with a strong emphasis on problem solving using cutting-edge tools and techniques culled from a wide range of disciplines. This Photonics/Materials laboratory can be regarded as an integration of laser optics equipment normally found in a physics department, with thin film and surface science analysis techniques normally found in a chemistry department, to address research challenges of materials engineering.
Within the Department of Chemistry at McGill, the Barrett group has created collaborative research projects in the in the areas of surface characterization, self-assembly, and polymer synthesis, with Professors Lennox, Eisenberg, Sleiman, and van de Ven. Collaborations with the NMR spectroscopy group headed by Prof. Reven have already been initiated, with a shared undergraduate honours project student and technician co-hired to work on joint projects. Extra-departmentally, the Barrett group plans to forge similar sustained research partnerships, including the Grutter group in Physics, and the Wood-Adams group in Chemical Engineering. The research team is a member of the McGill FCAR Centre for the Physics of Materials, comprised of 9 members of the Physics Department and 4 from Chemistry and Hydro-Quebec. The Barrett group will be a co-user of new infrastructure in the Department of Physics at McGill for nanostructural science tools, and plans to lead the Chemistry contingent into a newly-created materials partnership at McGill housed in the new Wong Engineering Building.
Specific program of research
The goal of this research program is to investigate the extent to which one can probe and control the physical properties of polymer thin films with light, through the incorporation of photo-addressable functional groups. Insight into this research challenge will advance our understanding of the materials used for applications such as bio-compatibility, molecular recognition, and next-generation information storage and processing. While criteria for specific applications (such as electro-optics or bio-activity) serve to motivate the proposed approach, the research goals lie just as much in an investigation into the fundamental nature of these materials and structures, and their interactions with light. Through these studies, two research challenges will be addressed: probing the delicate in situ structure of polymeric layers self-assembled in an aqueous environment, and controlling aspects of this structure and conformation reversibly and externally with laser light. While this technique of photo-control over bulk properties has been well studied in other polymeric media, the novelty of this proposed research lies in the hydrophilic nature of the materials—permitting the compatibility of current information processing, storage, and signaling technologies directly with biological systems.
This research is multi-disciplinary in scope, reaching from physical polymer chemistry into optical physics, surface science, and bio-engineering, providing an opportunity to make valuable contributions to a number of different areas of research. These areas include polymer synthesis and characterization, thin film fabrication, surface science, optical physics and spectroscopy, photochemistry and photophysics, and electo-optic device fabrication. The optical experiments described here are not routine in any sense, but rather use an individually designed and assembled setup for each experiment. This design-from-scratch approach using basic optical components thus provides one of the best possible opportunities for practical education of the researchers involved, as the intellectual exercise of critically designing and conducting most aspects of one's experiments is invaluable as basic academic and scientific training. Furthermore, this multi-disciplinary approach allows an excellent opportunity to advance knowledge into completely new research directions that can be either discovered or envisaged through the course of this research.
Course of research
The motivations for this research program stem from the dichotomy that exists between current technologies and natural systems, in their approach to construct functional materials. While the former class is the approach of preference at moderate lengthscales, there are appreciable difficulties in scaling down materials and methods to the micro- and nano-scale. In this regime, arguably some of the most successful functional systems (for example self-replication, sensing, recognition, and information processing) are inter- or intra-cellular natural systems, assembled in an aqueous environment. Lengthscale limits encountered recently by lithography, and defect tolerance barriers met by processing architectures for example, serve to illustrate the pressing need for an exploration of fundamentally new methods and materials. One attractive approach is to mimic the design of some natural systems (a recent overview is provided by Williams, Science 1999, 285, 391). The great challenge to reconcile the different natural and technological approaches is not just one of materials, as their interaction can also be quite dissimilar. For example, electric current, light and strong mechanical force are used in artificial systems, while surface shape, polarity, and weak intra-molecular forces (triggering molecular recognition) are preferred for natural. There is hence much value to investigating materials and methods which operate at the interface between natural and artificial systems, and of specific interest is how electric or light signals of current technologies can sense and modulate the shape or polarity of biological systems.

A breakthrough in the search for a water-based platform for electro-optic and bio-active materials was achieved in 1994, with the development of a technique to prepare multilayers of polyelectrolyte (PE). These films are assembled through the sequential and repeated adsorption of alternating layers of polycations and polyanions from dilute solution to build up, layer by layer, specialized structures of hundreds of nanometres in thickness.[1-3] Although this technique has been developed only recently, good stable films of uniform and controlled thickness can be produced, which have been shown to incorporate a variety of functionalities such as charge transport,[4] electroluminescence,[5] or nonlinear optical activity,[6] to form simple electro-optic (EO) devices. Of particular interest to this proposal is the aqueous assembly and hydrophilic nature of these devices, which allows compatibility with biological systems and function, as well as with any metal, organic, or inorganic devices mounted on the same platform. This sequential dipping method has been demonstrated to be suitable to a wide range of substrates (glass, Si, metals, etc.) and for any polymer which includes water-soluble ionic functionalities. This technique is also well-suited to forming small scale, 3-D films (in contrast to usual methods such as spin coating or vapour deposition which are limited to 2-D), and the coverage (lack of defects) is remarkably good. Despite much recent study however, many fundamental questions remain unanswered concerning the structure of the resulting layers. Information regarding the in situ conformation of the adsorbed layers is challenging to obtain, but is important to both the mechanistic study of the adsorption process, and for the insight that might be gained about more complex polyelectrolytes (such as proteins) adsorbed to surfaces.
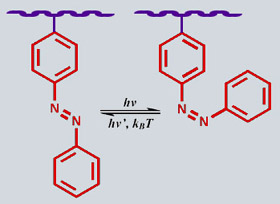
The photo-addressable functionalities which are incorporated into the polyelectrolyte chains are azobenzene-based (azo) chromophores. These undergo a rapid and reversible isomerization upon irradiation with visible light, and have been shown to be readily incorporated into a wide variety of polymeric systems.[7] These compounds are similar to other isomerizing chromophores such as rhodopsin or spiropyran, yet are superior as photo-switches due to the fast, complete, and clean conversions. Furthermore, the rates and extent of these isomerizations have been shown to be very sensitive to the local environment, suggesting their use as a probe.[8] There is a large geometric change associated with this photochemical conversion, and folding into the cis geometry from the more stable trans can be used to effect a significant and reversible change in structural, optical, electrochemical, or surface properties in a host material.[9-16] This program of research focuses on changes to the structure and surface of azo-containing aqueous systems, both in solution and in hydrophilic layers. For example, azo-functionalized polypeptides and cellulose polymers have been prepared by other research teams with a solubility in water that is photo-switchable,[9] and liposome membranes incorporating azobenzene exhibit transport properties which can be controlled by light.[10] Other interesting properties of aqueous systems which have been shown to be photo-switchable by azo-functionalization include the chirality of optically-active polymers,[12] and control of the twisting of helical polymers such as polypeptides,[13] and polyisocyanates.[14] These, and similar studies of azo-switched protein conformation and enzyme activity,[15,16] were conducted in dilute solution only, but demonstrate quite convincingly that primary, secondary, and even tertiary structure of polypeptides can be reversibly photo-switched with even a small amount (a few percent) of functionalized chromophore. However, this wide range of systems into which azobenzene has been incorporated as a reversible photo-switch has not yet included any hydrophilic surfaces, such as PE multilayers. It has been demonstrated recently that azo-functionalized PEs can be assembled into multilayer structures by sequential adsorption,[17] yet the chromophores were included only for their strong absorbance to monitor layer thickness, or for their nonlinear optical properties. To date there have been no attempts to investigate the direct reversible structural or surface changes that could be induced by irradiation of PE multilayer films with light.
The program of research to be conducted by the Barrett Group at McGill explores the use of azobenzene as both a "photo-probe" and as a "photo-tool" when incorporated into PE films. The novelty of this research program lies in the merging, for the first time, of the photo-switching capabilities of azobenzene and the hydrophilic properties of PE multilayers. A long-term goal (10-year timeframe) of this set of investigations is to demonstrate a two-way compatibility between traditional means of sensing and signaling (electric current or light) and natural pathways (shape or surface energy). Shorter-term (2-year timeframe) projects focus on the preparation and initial optical characterization of azobenzene-containing polyelectrolyte (azo PE). Two main avenues of investigation which can be detailed here for illustration represent both 'passive' and 'active' employment of the incorporated photo-addressable groups, where A) the chromophores are used as a structural probe, and B) the chromophores are employed as a structural tool. The first project describes how small quantities of azo PE can be selectively introduced into specific locations in the polymer film, and the photoisomerization behaviour monitored in situ to construct a concentration profile. A longer-term (5 year) goal of this project is to develop this as a simple and general technique to determine complex structural information in situ for a variety of organized media. In the second project described, moderate amounts of azo PE are incorporated into multilayers and surface properties are monitored during photoisomerization to assess how the chromophore exerts influence. A longer-term (5 year) goal of this project is to demonstrate light-control over surfaces with protein or cell receptor sites, to direct molecular recognition identity or cell growth. The specific projects described below have a 2-3 year timeframe:
1) Azo PEs as a Photo-Probe: This project will develop a new and elegant technique to determine the in situ concentration profile of polyelectrolyte structures assembled onto surfaces. This information is crucial both for investigating the mechanism of the multilayering process specifically, and in general for the determination of the structure of surface-adsorbed polymers. Measurements such as these are valuable, for example, in the areas of colloidal interactions and protein adsorption, where the in situ conformation can be markedly different than that in the dry state, where most measurement techniques are confined. Recent reports confirm that multilayers in water can swell substantially and nonuniformly,[18] yet the only current method for obtaining the in situ conformation profile is neutron reflectometry,[19] Due to the enormous time and expense required to run and analyze a single sample however, this technique becomes prohibitive for the large number of polymers and variables to be investigated. The approach to be taken here is to measure the rates and extent of the photochemical and thermal isomerization of azobenzene polymers layered into selective and localized regions of the multilayer film, to deduce structure. The thermal isomerization rate (reconversion in the dark to the cis form from photo-pumped trans form) has been shown to be especially sensitive to the local environment,[8] suggesting that it can be used as an aqueous probe. Typical half lives for the cis form can range from hours (in some polar solvents) to seconds (in bulk), and hence the nature of the local solvent environment can be deduced quantitatively. The first step will be to determine that the levels of incident light and azo loading are sufficiently low enough to prevent the isomerization from significantly disrupting the local PE structure to be probed. This will be accomplished by performing a series of rate determinations at various azo loading levels, over a range of laser intensities. Since the magnitude of a structural disturbance would be expected to scale with the intensity of the light used, the thresholds of intensity and azo loading can be determined as the levels below which the thermal isomerization rates are independent of irradiation level. The absorbance of most azo chromophores is strong enough (and quantum yields high enough) that even extremely low concentrations (layers < 10 nm) irradiated with low light levels (< 1 mW/mm2) will still provide sufficient signal to determine isomerization rates. This method will confirm that azo incorporation can be regarded as an unobtrusive and benign structural probe below these thresholds.
Films will be placed in an aqueous sample holder under the desired solution conditions, and exposed briefly to a beam from an argon ion or pumped diode laser near the absorbance maximum of the trans to cis conversion (usually 488, 514, or 532 nm). The adsorption will be measured with a second low-power laser beam, to monitor of the growth and decay of the cis population over time, and thus determine the extent and rates of the isomerizations. This spectroscopic characterization will be carried out 'free form' on an optical table with lasers and detectors of various wavelengths, for maximum sensitivity and versatility. Reference rates will be determined from azo PEs dissolved in solutions of controlled properties, and these compared to the rates measured in the swollen films. In this way, local ionic strength and acid/base equilibria can be probed at known locations inside the swollen structure, which can be strongly non-equilibrium and difficult to model. This will allow direct measurement of the local solvent environment, which represents information not currently obtainable by other techniques. After the initial work is able to identify the variables of greatest influence, further study will vary the molecular structure of the chromophore, to assemble structure/property relationships between the chromophore, isomerization behaviour, and the local environment. An eventual goal is the development of this probe technique as a general multi-variable in situ method suitable for a variety of swellable films or assemblies. With chromophores individually tuned for sensitivity to a range of solvents and solution conditions, one could envisage an array of azo probes being addressed and monitored independently yet simultaneously, forming a simple and elegant sensor.
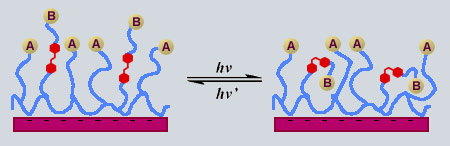
2) Azo PEs as a Photo-Tool: In this project the azo PEs (as prepared in A) are similarly assembled into select locations in PE layered structures, though to a much greater extent (ie: from 10s of nm to 100s of nm). First we will determine the extent to which we can modulate with light the surface properties (wettability, chain conformation, mobility, and adhesion) of these thin films. This will be accomplished by exciting the chromophores near the absorbance maximum to induce isomerization, and simultaneously measuring bulk and surface properties by methods including contact angle, ellipsometry, and surfaces forces. The influence of induced motion will thus be determined as a function of light intensity, chromophore content, and distance away from the s urface that the azo PE is placed. An emphasis will be also placed on relating the observed changes to the molecular structure of the chromophores. As a compliment t o these macroscopic measurements, we will also investigate these effects with solid state NMR spectroscopy. Techniques for multilayering PEs onto colloids have been demonstrated recently,[20] which for the first time will allow an opportunity to directly probe the chain motion in multilayers (in collaboration with Prof. L. Reven at McGill). PE multilayers will be deposited onto 70nm silica particles, and we will measure the light-induced changes in motion, conformation, and density of the chains. After the extent of the influence of isomerization has been determined, research will focus on surfaces supporting bio-active functionalities. Recent studies of self-assembled monolayers end-capped with a variety of peptide sequences (such as RGD) optimized to promote (or inhibit) protein and cell adhesion has been reported.[21] This provides published preparation techniques for co-polymerizing a series of peptide end-capped monomers with acrylic acid monomers to provide bio-active PEs, which will be layered on top of azo PEs. Photo-control over the bio-activity of the surfaces of these films will then be measured, by observing cell attachment and spreading, in the presence or absence of light. Further studies will use surfaces functionalized with both peptide and azo sidegroups ('A' and 'B' respectively in the scheme depicted). Here the surface properties will be monitored (such as contact angle) during irradiation, with the goal of developing a reversible photo-switchable surface identity between a predominantly B and predominantly A character. A and B will be varied to also include pairs such as -OH and -CH3 (adsorbing and inert), -RGD and -PEO (bio-active and -inactive), -COOH and -CF2CF3 (wet and dry) and -COOH and -NH3 (acidic and basic). Furthermore, if probed as per the previous project, the isomerization rates of the azo groups in the vicinity of the bio-active surface can be examined as a function of adsorbant, to explore the extent to which these chromophores can 'recognize' specific proteins or cells adsorbed on the film surface, resulting in a specific spectral signature for different adsorbants.
REFERENCES
1.Decher and Lvov, Thin Solid Films 1994, 244, 772.
2.Ferreira and Rubner, Thin Solid Films 1994, 244, 806.
3.Decher, Science 1997, 277, 1232.
4.Cheung and Rubner, Macromolecules 1998, 30, 2712.
5.Fou and Rubner, J. Appl. Phys. 1996, 79, 7501.
6.Wang and Tripathy, Macromol. Chem., Rapid Commun. 1997, 18, 451.
7.Xie and Natansohn, Chem. Mater. 1993, 5, 403.
8.Barrett and Natansohn, Macromolecules 1994, 27, 4781.
9.Arai and Kawabata, Macromol. Chem., Rapid Commun. 1995, 16, 875.
10.Kano and Kunitake, Chem. Lett. 1980, 421.
11.Wurthner and Rebek, Angew., Chem. Intl. Ed. Engl. 1995, 34, 446.
12.Chen and Wang, J. Poly. Sci. A 1997, 35, 9.
13.Sisido and Tazuke, Macromolecules 1995, 28, 4981.
14.Muller and Zentel, Macromolecules 1996, 29, 1609.
15.Willner and Rubin, S. Angew. Chem. Intl. Ed. Engl. 1996, 35, 367.
16.Morishima and Seki, Macromolecules 1993, 26, 3299.
17.Yang and Tripathy, Appl. Phys. Lett. 1998, 73, 3345.
18.Farhat and Schlenoff, Langmuir 1999, 15, 6621.
19.Kellogg and Mayes, Langmuir 1996, 12, 5019.
20.Caruso and Mohwald, Macromolecules 1999, 32, 2317.
21.Lauffenburger and Whitesides, Biomaterials 1999, 20, 1213.