Nanotechnology:
A Brief Overview
| SELF-ASSEMBLY | |
|
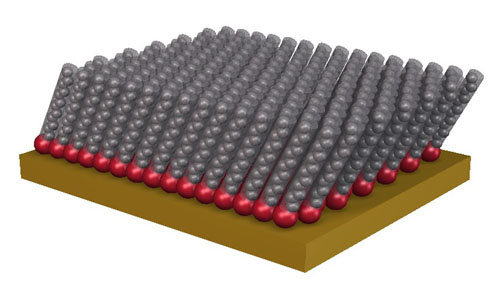
For the bottom-up approach to work, we need some way to take basic building blocks (such as molecules) and build them into larger units. One of the most promising paradigms for accomplishing this is self-assembly. In traditional assembly, the individual pieces are constructed, and then are physical put together, one piece at a time, in a painstaking and serial process. In self-assembly, the starting materials are cleverly designed so that they automatically, spontaneously form the desired aggregate structure. This obviously simplifies assembly, since no external direction is required: the pieces do all the work. Of course, designing starting materials cleverly enough to assemble properly is no simple task. In actual examples in the literature, self-assembly has a slightly narrower definition. It is specifically the spontaneous (usually thermodynamic) formation of structures due to complementary chemical affinities in the various starting materials. For instance, block copolymers form a variety of self-assembled morphologies in solution. A copolymer with a short hydrophobic block and long hydrophilic block, in water, would form small micelles, with hydrophobic cores, surrounded by extended hydrophilic coronas. The structure is produced because of the energy-minimization that occurs, where the hydrophobic portions would rather aggregate, whereas the hydrophilic portions would rather be solubilized. Self-assembly is also what allows a cell wall (lipid bilayer), and other biological vesicles to form. Another classic example from the literature is self-assembled monolayers (SAMs). The prototypical SAM is an alkane-thiol chain which is allowed to adsorb onto a gold surface. The affinity of sulfur (red in the figure) for gold attaches the chains onto the surface, and the strong alkane chain interactions cause them to form fairly well ordered, tilted layer structures.
Self-assembly is also responsible for the efficient folding of proteins into their unique solution-phase structure. In this case, intra-molecular complementary chemical functionalities, and hydrophobic-hydrophilic interactions, cause the polymer to adopt one preferred conformation, which is then responsible for the protein's specific function. Self-assembly, in a sense, is also responsible for the tight binding (and specificity) of the DNA double helix. Self-assembly is thus able to do many things. It can form ordered arrays on substrates (such as SAMs or photonic crystals). It can create very complex molecular structures (such as proteins). It can form precise molecular aggregates (as in the case of many biological structures, organelles, and cells). It can be used to selectively position aggregates on substrates. Essentially, it is hoped that self-assembly will allow for nano-sized devices to be built by assembling carefully designed starting materials. Then, these aggregates can be selectively positioned on substrates (if this is desired).
|
|
| SELF-ASSEMBLY APPLICATION | |
|
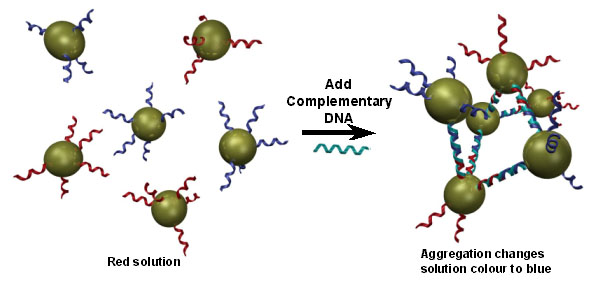
To illustrate the concept of self-assembly, consider the following example from recent literature. Gold nano-particles, functionalized with two specific sequences of DNA, are dispersed into solution (which becomes red). When DNA which is complementary to both sequences is introduced, it binds the two sequences, linking previously disjoint gold nanoparticles. This causes the gold particles to form aggregates in solution. The aggregates change the optical properties of the solution, making it turn blue. This has been proposed as a rapid and simple method of verifying the presence of a given nucleotide sequence. Target material can be added to a solution of nanoparticles bearing functionalities complementary to the target strand. If the target DNA is present, a solution colour change (easily detectable by the naked eye) will signal it.(1)
While this may indeed lead to faster, field-usable, and reusable, DNA identification, it also highlights the power of self-assembly techniques.
|
|
| SELF-ASSEMBLY FABRICATION | |
|
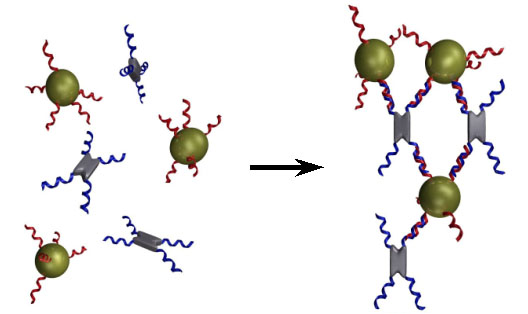
The same biologically-derived self assembly previously described (based on complementary DNA sequences) can be exploited to build nanometre-sized devices, at least in theory. The concept is to exploit DNA's very specific binding to control the aggregation of sub-units. In theory this would allow for the easy tailoring of what sub pieces bind to which, and in what ways.(2)
|
|
| REFERENCES | |
|
|
|
|
|