|
|
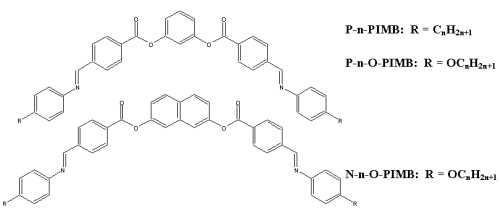
A
peculiar LC system was discovered recently, which, contrary to
predictions, displayed ferroelectric switching (which usually
only occurs in chiral LC systems).(1) Banana-shaped mesogens (shown
above), first synthesized by Matsunaga and coworkers(2,3), were
found to form macroscopically chiral domains. Clearly some form
of spontaneous symmetry breaking was responsible for the formation
of chiral domains, since the constituent molecules have no intrinsic
chirality. The handedness of the domains was random (the left
and right-handed versions were equally likely), but one handedness
could be formed preferentially with the addition of a small amount
of chiral dopant.(4)
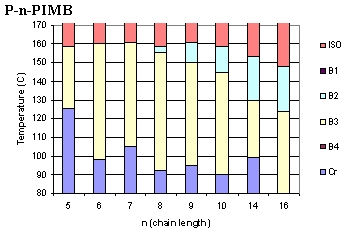
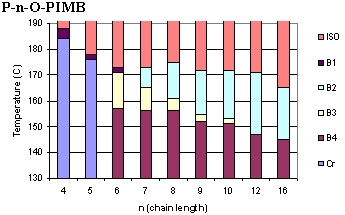
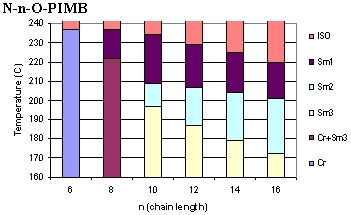
The
banana mesogens form a number of phases, shown to the left.(4-7)
At low temperature, the banana liquid crystals display the usual
crystalline (Cr) phase, and at high temperature melt, as expected,
into isotropic liquids (ISO). The B-phases (as they have come
to be called) are unlike what is found in other LC systems, since
they exhibit many chiral properties, although the banana mesogens
are not, themselves, chiral (in stark contradiction to conventional
LC wisdom).
|
|
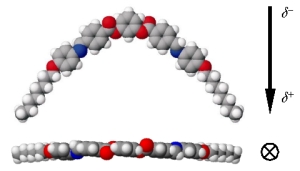
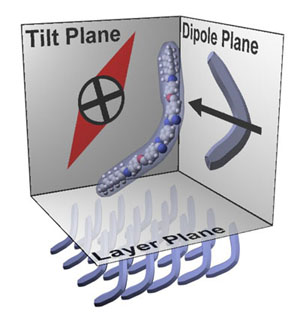
How
can an achiral mesogen form a chiral phase? The exact structure
of the phase took some time to elucidate. Eventually, however,
it was understood how a chiral coordinate system could be defined
from polar banana-shaped molecules (the optimized molecular structure,
along with orientation of dipole, is shown to the right). The
mesogens form a layered structure similar to the smectic C phase,
with mesogens tilted with respect to the layer normal. The layer
normal, dipole vector, and tilt direction (or equivalently, the
layer plane, tilt plane and dipole plane) form a coordinate system
(since they are represent three non-coplanar vectors, hence define
a basis). The handedness of the coordinate system is based upon
the sign of the tilt. Thus, each smectic-like layer of bent polar
molecules is chiral.
|
|
 |
The
B2 phase is formed from alternating layers of such chiral smectic
layers. Two textures are found in the B2 phase, termed Racemic
and Homogeneous. The racemic texture has layers which alternate
in handedness (subsequent layers have opposite dipole orientation).
The homogeneous phase is completely chiral: subsequent layers
have opposite dipole orientation and tilt, hence same overall
handedness. Each domain of this texture is thus a homogeneously
chiral region.
The
polar molecules can be oriented in an electric field, with the
layers maintaining their handedness during electrical switching.
The B2 phase is thus antiferroelectric in the ground state, and
can be ordered to the ferroelectric state with an external electric
field (refer to the diagram which follows).
|
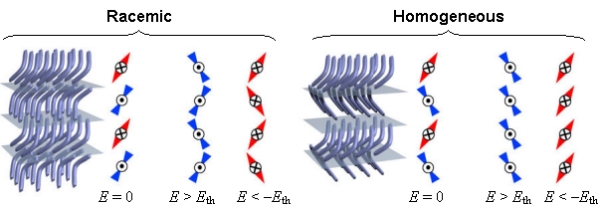
The
chiral domains of opposite handedness (for both he racemic and homogeneous
texture) were formed with equal probability. Application of appropriate
boundary conditions (such as pinning surfaces and applied electric field)
can make one chirality preferred. Also, addition of a small amount of
chiral dopant also makes one handedness lower in energy. Thus, a very
small amount of molecular chirality can be amplified, by the efficient
ordering of LCs, into macroscopically chiral fluids and solids (which
has obvious applications in chiral synthesis, chemical separations,
etc.).
The
B4 phase does not exhibit the layered structure found in the B2 phase,
but is still found to be chiral (although no longer antiferroelectric).(7)
In this case, it appears that a chiral helix is formed, running along
the direction of the layers.
|
1.Niori,
T.; Sekine, T.; Watanabe, J.; Furukawa, T.; Takezoe, H. J. Mater.
Chem. 1996, 6, 1231.
2.Matsunaga, Y.; Miyamoto, S. Mol. Cryst. Liq. Cryst. Sci. Technol.,
Sect A 1994, 237, 311.
3.Akutagawa, T.; Matsunaga, Y.; Yashuhara, K. Liq. Cryst.
1994, 17, 659.
4.Link, D.R.; Natale, G.; Shao, R.; Maclennan, J.E.; Clark, N.A.;
Korblova, E.; Walba, D.M. Science 1997, 278,
1924.
5.Jakli, A.; Rauch, S.; Lotzsch, D.; Heppke, G. Phys. Rev. E
1998, 57, 6737.
6.Thisayukta, J.; Nakayama, Y.; Kawauchi, S.; Takezoe, H.; Watanabe,
J. J. Am. Chem. Soc. 2000, 122, 7441.
7.Thisayukta, J.; Takezoe, H.; Watanabe, J. Jpn. J. Appl. Phys.
2001, 40, 3277.
|






