|
Surfactants
(soap molecules) can form a variety of liquid crystalline phases
in water. This ordering is due to hydrophobic/hydrophilic competitions.
The hydrophilic (ionic) portion of the surfactant is more stable
when solubilized by water, whereas the hydrophobic (alkane) portion
of the surfactant is more stable when surrounded by other organic
chains. Hence, the materials self-assemble in the liquid phase,
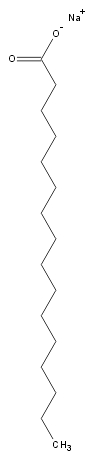
giving it a higher than isotropic order. The structure of a typical
surfactant (sodium laurate) is shown on the right. Below is a
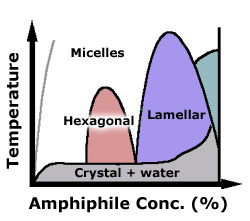
typical phase diagram for a surfactant, as a function of concentration
(in water) and temperature. Amphiphiles have rich phase diagrams,
with many novel morphologies. Schematics of some phases are shown
below.
|
 |
|
In
dilute solution, the surfactants do not form any particular structure.
As the concentration is increased, however, the amphiphiles condense
into well defined structures.
|
 |
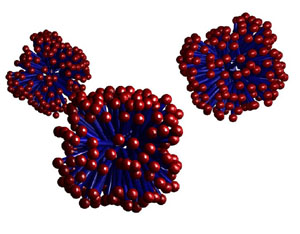 |
The
most readily formed structure is micelles, where the surfactants
hide the hydrophobic tails inside a sphere, leaving only the water-soluble
ionic heads exposed to solution.
|
|
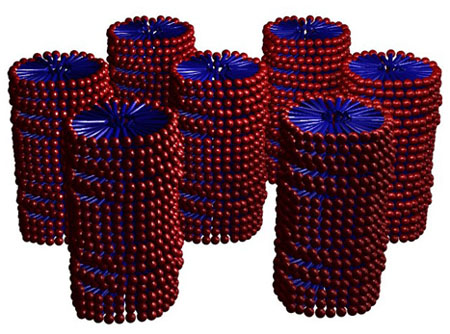
At
higher concentration, surfactants can also form elongated columns
that pack into hexagonal arrays. The columns have hydrophobic
cores and hydrophilic surfaces. The columns are separated from
one another by water.
|
 |
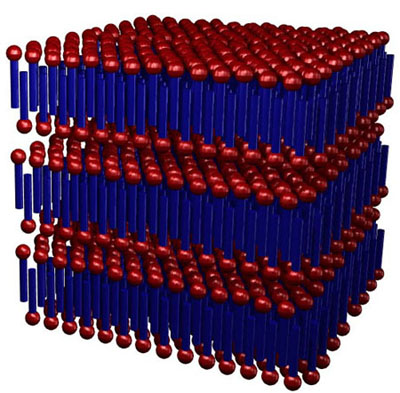 |
At
extremely high concentration (neat soap), the surfactants crystallize
into a lamellar structure, with elongated sheets separated by
thin water layers. The structure is very reminiscent of the lipid
bilayers seen in biological systems.
|
|