Introduction
to Liquid Crystals
| WHAT IS A LIQUID CRYSTAL? | ||||
|
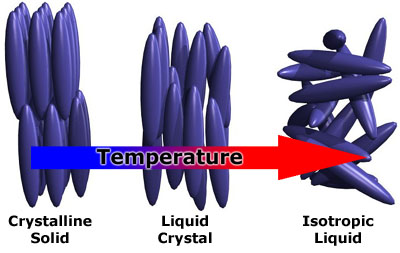
The common states of matter, namely solid, liquid and gas, differ mainly by the types of and degree of order present in the phase. These states of matter, however, are not sufficient to characterize the structures found in all systems. As most substances are heated, they go from a solid (usually crystalline, possessing high order) to an isotropic liquid (highly disordered). Some substances, however, exhibit intermediate states lacking some of the order found in solids, but possessing more order than found in liquids. These ordered fluids are called liquid crystals. Crystalline solids have positional and orientational order. Conventional liquids have neither. A liquid crystals, on the other hand, might have no positional order, but some orientational order (with correlations between the direction of neighboring molecules). This ordering usually persists only for a fairly narrow temperature range, and is related to the intrinsic molecular shape. Liquid crystalline molecules, called mesogens, are usually highly anisotropic in shape, which gives rise the preferred orientations of nearby molecules. For illustrative purposes, let us begin by looking at some of the phases observed for rod-like mesogens.
|
||||
| NEMATIC PHASE | ||||
 |
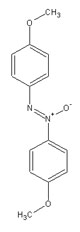 |
The nematic phase has no positional order, but has orientational order. That is, the mesogens all point in the same direction, essentially expressing the molecular anisotropy as a phase anisotropy. A given molecule's orientation is not constant, and all the mesogens do not point in exactly the same direction. For an isotropic liquid, averaging molecular orientations gives no result, since there are as many molecules lying along one axis as another. In the nematic phase, averaging molecular orientations gives a definite preferred direction, which is referred to as the director. It is important to remember that liquid crystals are liquids, meaning that although there is an average order, molecules are constantly flowing, and moving, changing position and orientation. The structure of p-azoxyanisole, a low molar mass liquid crystal exhibiting a nematic phase, is shown. A schematic representing mesogens as ellipsoidal rods, oriented in roughly the same direction, shows conceptually what order in the nematic phase is like
|
||
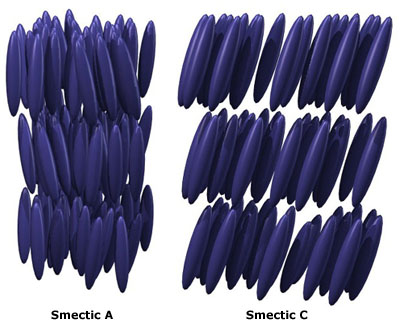
| SMECTIC PHASES | ||||
|
||||
|
|
||||